ISO 11239 IDMP Standard lists regulated information on pharmaceutical dose forms, units of presentation, routes of administration and packaging.
The ISO 11239 standard identifies for example injection solution, injection suspension, infusion solution (or a less granular regional term linked to these.
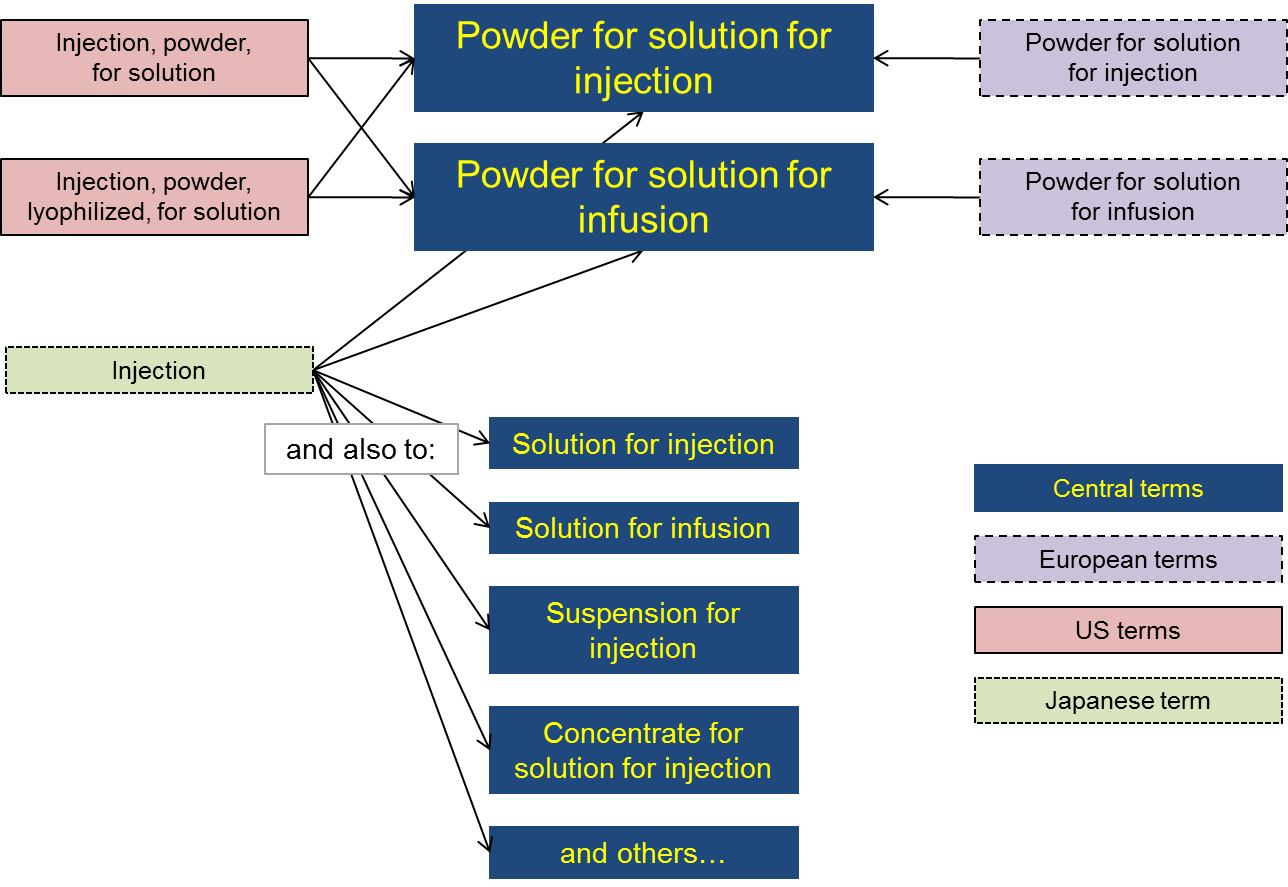
Differences in granularity between regional terminologies
Many of the concepts covered in ISO 11239 are already described in various regions using their own sets of terminologies, which are usually not harmonised with those of other regions. This is often because of different levels of granularity between regions, which means that one-to-one mapping between those regions’ terms is not possible. For example, if the terms used in region A have a relatively low level of granularity compared to the terms used in region B, one term from region A will often map to several terms from region B. Indeed, the purpose of ISO 11239 is to allow for the development of a single set of controlled vocabularies that can be used by all regions to communicate with each other, and to which all regions can map their own regional terms.
The figure gives an example of how terms from Europe, the US and Japan might map to a list of central terms.
In this example:
- central terms distinguish between injection and infusion products but not between lyophilised and non-lyophilised powders;
- Europe distinguishes between injection and infusion products but not between lyophilised and non-lyophilised powders (i.e. the same as the central terms);
- the US distinguishes between lyophilised and non-lyophilised powders but not between injection and infusion products;
- Japan uses a single term to describe all injection and infusion products.
As a result, with respect to the central terms there is one-to-one mapping for each European term, there is one-to-two mapping for each US term, and there is one-to-many mapping for the Japanese term.
ISO 11239 Screen from the IDMP Term Browser® for “capsule”:
Capsule ( EDQM FDA EMA )
Synonym and Abbreviations:
Kapsel
Kapsula
Kapsulė
Kapsulë
Капсула
Капсули
Synonym Details:
| Term | Lang | Role | Code | Type | Source |
|---|---|---|---|---|---|
| Capsule | EN | PT | 12100 | SYN | EDQM |
| Capsule | EN | PT | C25158 | PHF | FDA |
| Capsule | EN | PT | PHF00005MIG | PHF | EMA |
| Kapsel | ET | ST | 12100 | SYN | EDQM |
| Kapsula | SR | ST | 12100 | SYN | EDQM |
| Kapsulė | LT | ST | 12100 | SYN | EDQM |
| Kapsulë | SQ | ST | 12100 | SYN | EDQM |
| Капсула | KK | ST | 12100 | SYN | EDQM |
| Капсули | UK | ST | 12100 | SYN | EDQM |
Other Properties:
| Type | Code | Descr |
|---|---|---|
| Contributing Source | EDQM FDA EMA | |
| Semantic Type | SYN PHF PAC PRE |