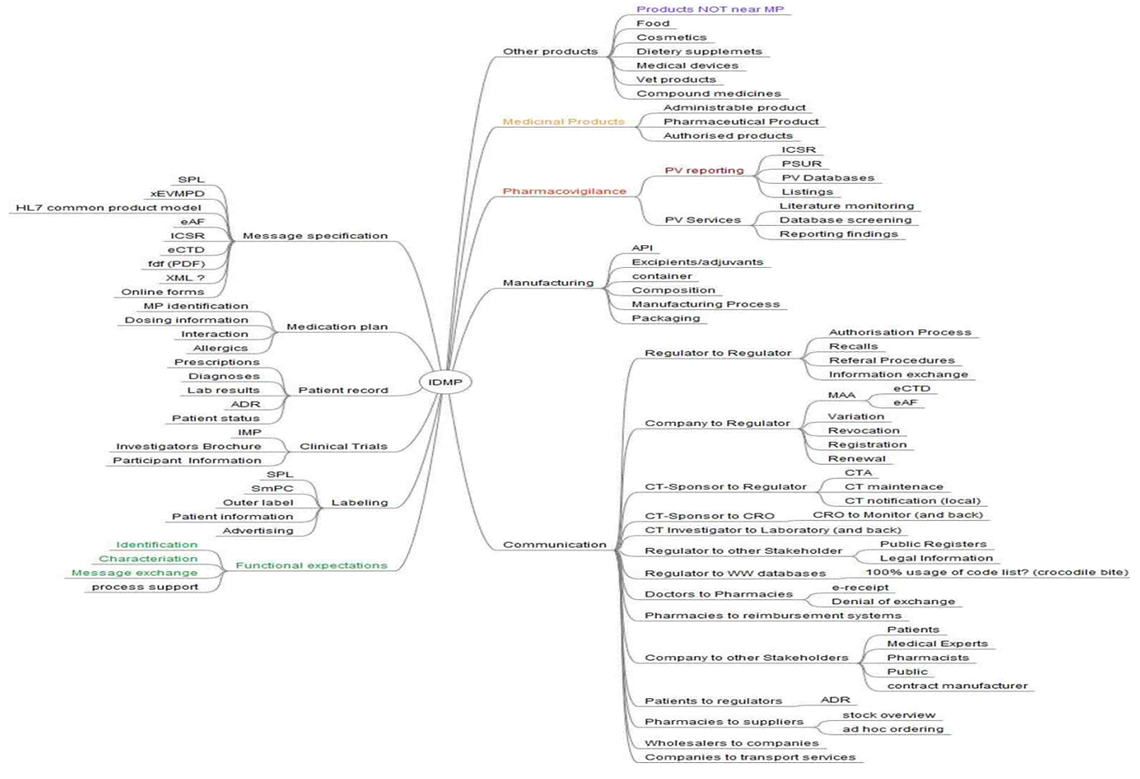
IDMP is aimed to be used wide out of the regulatory silo. Actually it will close the circle between the regulatory and the e-health world. This is the tremendous value of IDMP for the health care market worldwide. IDMP is the base for identifying medicinal products uniquely across all stakeholders.
IDMP Stakeholder Mindmap
IDMP was first aimed to support Pharmacovigilance (PV) reporting for ICSR, PSUR, and PV Databases. However IDMP will affect many more areas, processes and stakeholders in the health care market:
- the identifying of products not near Medicinal Products (MP), cosmetics, dietery supplements, vet products, and medical devices
- the identifying of aministrable product, pharmaceutical product, authorised products and Investigational Medicinal Products (IMP)
- the communication in Clinical Trials (CT) and Clinical Trials Application (CTA) – e.g. CT-Sponsor to the Contract Research Organisation (CRO), and regulator to World Wide (WW) Databases, patients to regulator concerning Adverse Drug Reactions (ADR) and many more
- the labeling (e.g. SPL and SmPC)
- the messaging (e.g. XEVMPD, HL7, SPL, ICSR, eCTD)
- the patient record and even the medication plan
This IDMP Stakeholder Mindmap has been prepared by Andreas Franken (Bundesverband der Arzneimittel-Hersteller e.V. BAH, Germany)