IDMP TERM BROWSER
MORE THAN 1 MILLION TERMS USED TO IDENTIFY MEDICINAL PRODUCTS WORLDWIDE
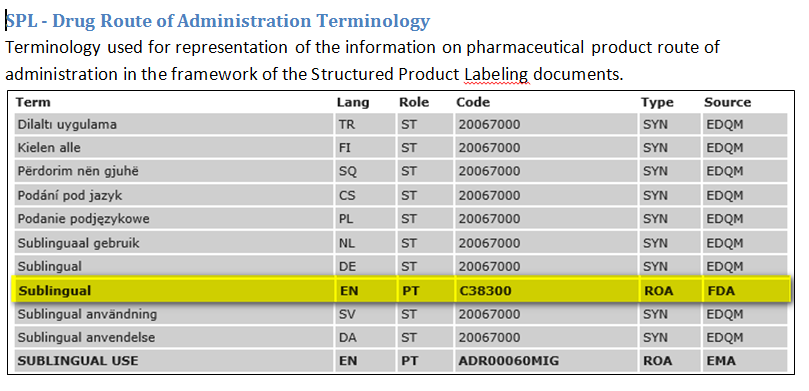
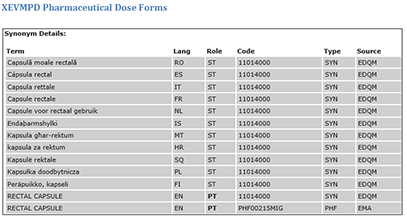
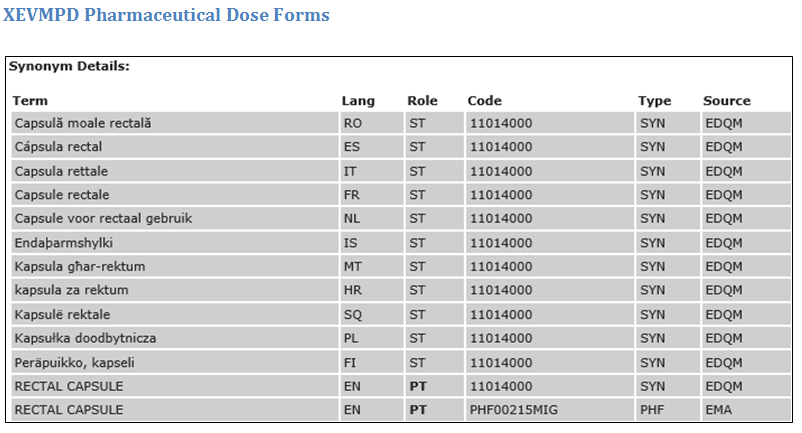
The IDMP Term Browser offers an overview to the encoding of referentials such as substances, indications, contra-indications, units of measurement, pharmaceutical dose forms, routes of administration etc.
THE ENCODING IS COMPLIANT TO ISO IDMP 11238, ISO IDMP 11239 AND ISO IDMP 11240 STANDARD
Using a smart and dynamic access to required external databases such as GInAS, UCUM and EDQM MedDRA, SNOMED, ATC (WHO), SPL, XEVMPD, UCUM, EDQM and GINAS.
ALL CORE FEATURES OF THE IDMP TERM BROWSER
Identify equivalent concepts in disparate terminologies
Check the semantic heterogeneity of concepts required within different legislations
Speed up manual identification of equivalent terms and codes
Integrate disparate data sources in your regulatory data
DIFFERENCES IN NAMING AND GRANULARITY OF CONTROLLED VOCABULARIES ON THE FIRST SIGHT
US: Aspirin
Europe: Acetylic Acid
US: lyophilisied or non-lyophilisied pulver (no differentiation between Injection and Infusion
Europe: Injection and Infusion (no differentiation between lyophilisied or non-lyophilisied pulver
"For me as CRO the IDMP Term Browser is the best way to find For me as CRO the IDMP Text Encoder is a revolutionary tool to increase adverse event specification to enhance the outcome quality of clinical trials."
Christine Klipping
President Dinox
President Dinox