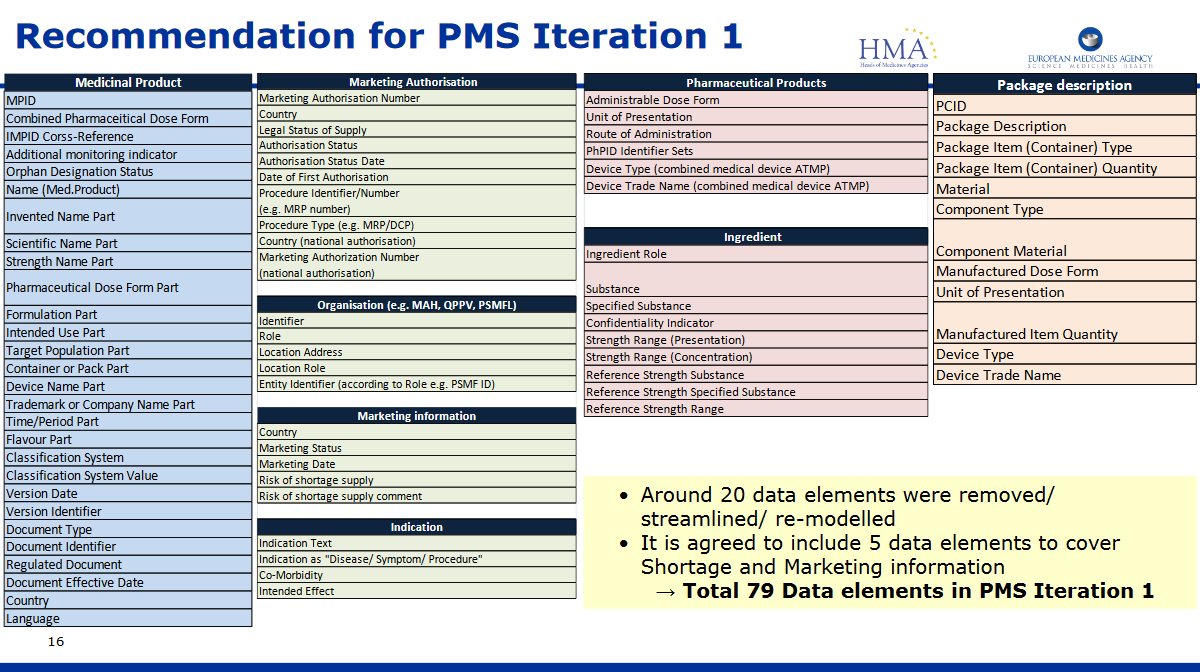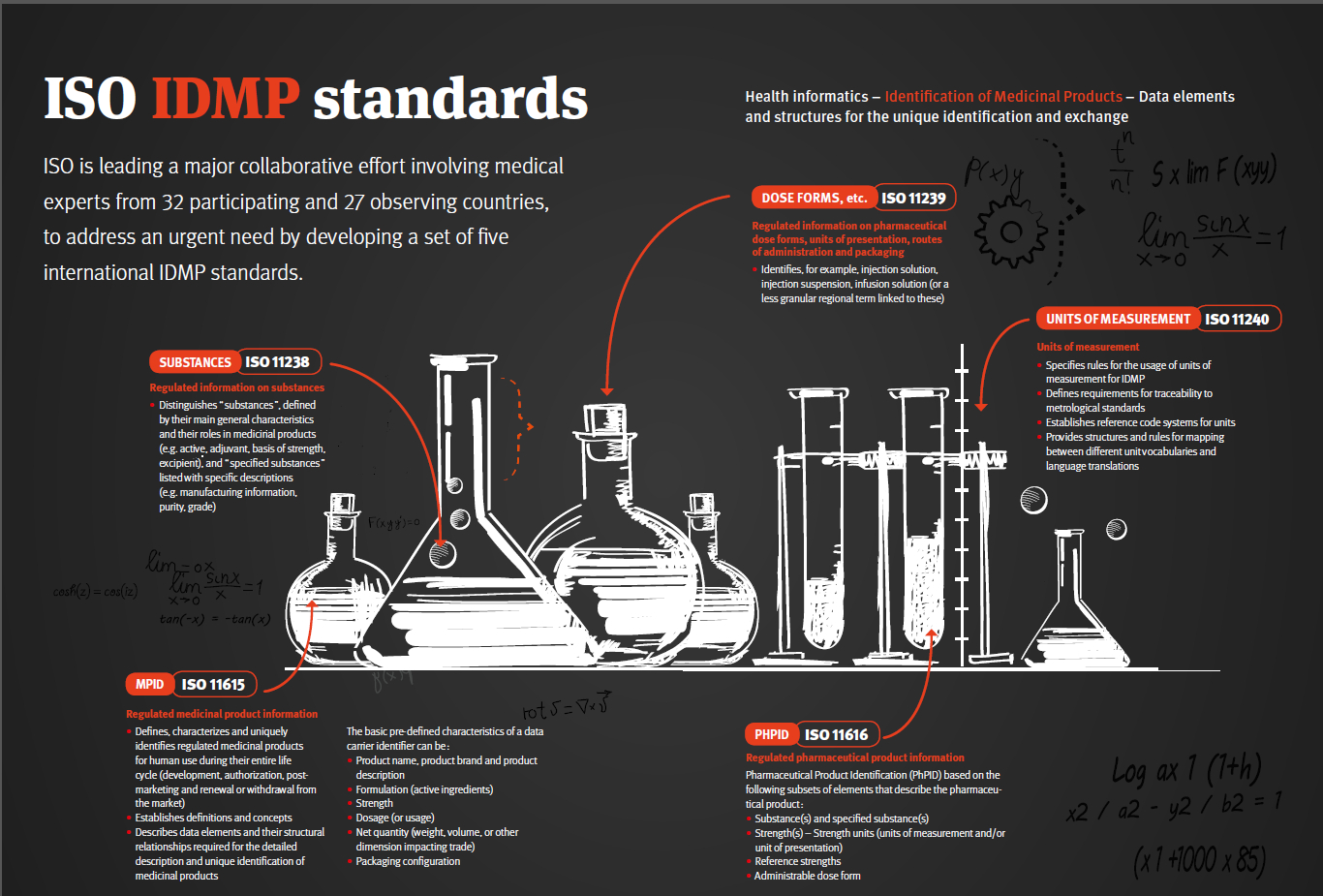
ISO Focus IDMP Article
ISO Focus ISO Focus is the official magazin of the ISO Organisation. Published in English, French and Spanish six times per year, ISOfocus is the gateway to International Standards. Whether a multinational enterprise faced with major decisions or a small business...