IDMP Gap Analysis
It is important to create a common understanding for the project of an IDMP Gap Analysis across the company. So typically all departments should be involved.
How can an IDMP Gap Analysis help you make your data management a success?
IDMP defines a precise structur for medicinal products mapped with controlled vocabularies.
The steps for the IDMP Gap Analysis could be:
- Identify on a very high level the data sources and the owners that are candidates for extraction of idmp required data
- Define some test products which offers variations in product type, substances (mono, combi with different strength), in-licensed products etc., old new products, centralised, mutual recognition procedure, decentralised procedure, and national procedure to be encoded and structured in IDMP. It is also advisable to use some local language SPCs for national products as they will have to be aligned with the IDMP data.
- Extract the required data manually out of the identified sources (step 1) and check if all needed information is available. Classify the quality of the extracted data in effort categories such as:
a. single source which can be mapped to IDMP
b. single source which requires significant effort to format data for mapping to IDMP
c. available in multiple systems requiring harmonisation, or with poor data quality
d. unstructured Data (Documentation)
e. location not found; substantial manual effort to retrieve information - Define (based on the outcome of step 3) your user requirements and potential high-level solution architecture. This can vary from an inhouse solution up to a wide range of solution scenarios with or without an integrated Master Data Management system
- The final step is the Request For Proposal (RFP) and the vendor selection and implementation
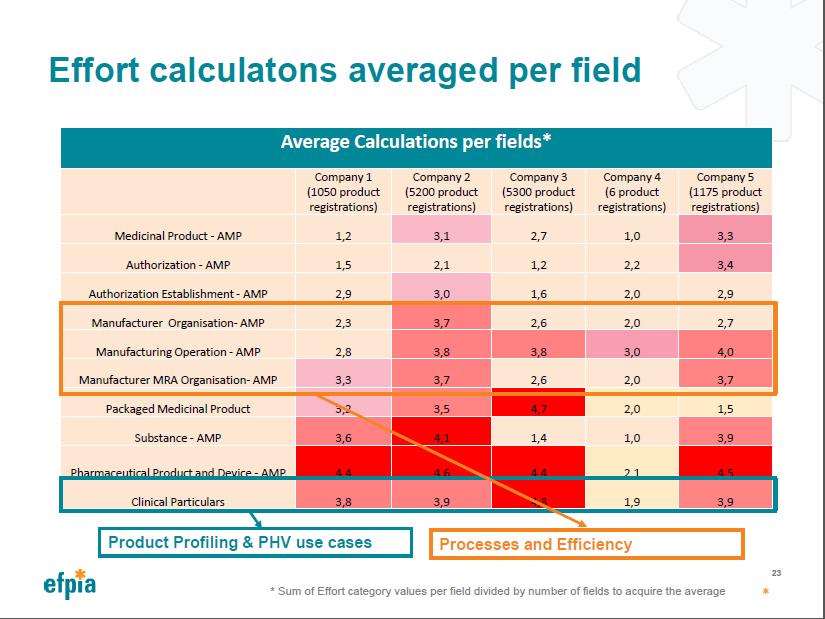
Overview of Industry Effort Calculation
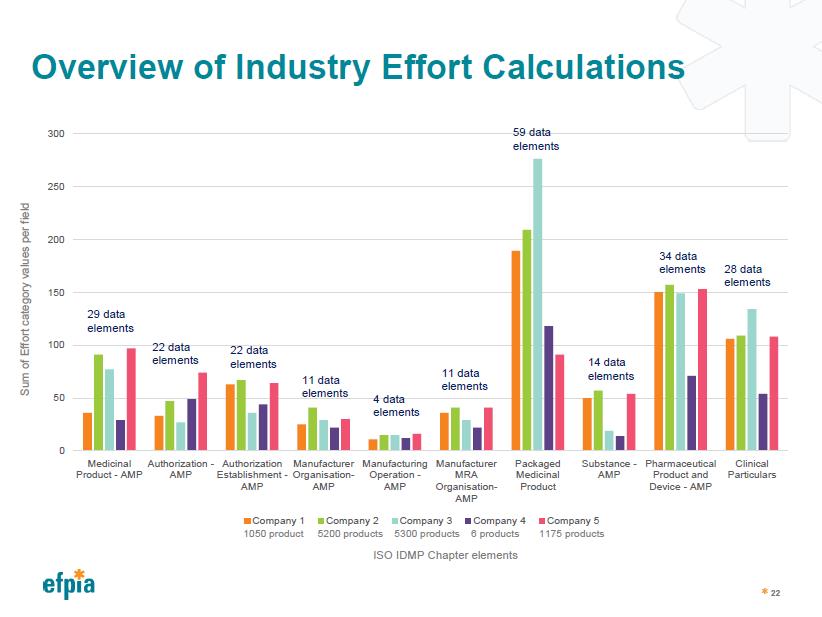
Effort Calculations Averaged per Field